
![069 - pH and BuffersIn this video Paul Andersen explains how buffer solutions maintain pH in a solution. A buffer solution is made up of a weak acid and its... Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and ...](https://i.ytimg.com/vi/FOjYUMIpor0/default.jpg)







![069 - pH and BuffersIn this video Paul Andersen explains how buffer solutions maintain pH in a solution. A buffer solution is made up of a weak acid and its... Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and ...](https://i.ytimg.com/vi/FOjYUMIpor0/default.jpg)







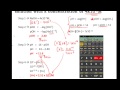
In the reaction NH3 (aq) + H2O (l) --> NH4+(aq) + OH- (aq), NH3 is the weak base, but does that mean that H2O is considered an acid because it donates its proton to form OH-? Top. Chem_Mod Posts: 19301 Joined: Thu Aug 04, 2011 8:53 pm Has upvoted: 856 times. Re: NH3 (aq) + H2O (l) --> NH4+(aq) + OH- (aq), acid and bas. Post by Chem_Mod » Sun Aug 21, 2011 6:47 pm . Yes. Remember water can act ... Answer to Give the conjugate base to each of the following species regarded as acids.a. HSeO4−b. PH4+c. HS−d. HOCl. A) H2O can form hydrogen bonds while CH4 cannot. B) H2O forms a less stable conjugate base, HO-. C) CH4 forms a more stable conjugate base, CH3-. D) H2O forms a more stable conjugate base, HO-. Page 5 In both instances, mass and charge are conserved. If we add a proton to water we get #H_3O^+#, the hydronium ion, which is an hypothetical species but is nevertheless useful for acid/base calculations.. Given this, you know that the conjugate base for water is #OH^-#, the hydroxide ion, and its conjugate acid is the hydronium ion, #H_3O^+#. The conjugate base of HSO4- is A) H2SO4 B) SO42-C) H3SO4+ D) HSO4+ E) OH-2) B. Sodium hydroxide is a strong base. This means that _____. A) aqueous solutions of NaOH contain equal concentrations of H+ (aq) and OH- (aq) B) NaOH does not dissociate at all when it is dissolved in water C) NaOH dissociates completely to Na+(aq) and OH-(aq) when it dissolves in water D) NaOH cannot be neutralized ... 1 decade ago. Favorite Answer. HC2O4- + H2O <----> C2O42- + H3O+ ( it acts as an acid) HC2O4- + H2O <-----> H2C2O4 + OH- ( it acts as a base) H2C2O4 is the conjugate acid. C2O42- is the conjugate... so HCO3- and CO32- is one such pair in which HCO3- is the acid and CO32- is the conjugate base. and another pair will be H2O and H3O+ ..in which H2O is the base and H3O+ is the conjugate acid. feel free to ask any question. 0 1. Anonymous. 9 years ago. EASY. Conjugate pairs are quite easy to identify -- they are the same molecule, but the difference between them is 1 hydrogen atom. Hence ... here H2CO3 and HCO3- are conjugate acid-base pair as are H2O and OH-b) HCl + H 2 PO 4-<-----> Cl - + H 3 PO 4. HCl transfers a proton (H+ ion) to H 2 PO 4- and form Cl - and H 3 PO 4, now Cl - can accept a proton donated by H 3 PO 4 .so the above equation is; HCl + H 2 PO 4-<-----> Cl - + H 3 PO 4. acid base base acid. conjugate acid-base pairs are HCl - Cl- and H 2 PO 4- & H 3 PO 4 . Similar ... View this answer. The conjugate base of H 2 O is OH -1, which is the polyatomic ion hydroxide. When we remove a hydrogen ion (proton) from a conjugate acid, the result... See full answer below. 28. What is the conjugate base of HNO in the following equation? HNO2 + H20 — H2O + NO," a) H30* b) H,0 c) HNO2 d) NO, - UO2 29. What is the pH of a solution whose concentration is 4.0 x 10-9 a) 4.00 b) 8.40 c) 3.60 d) 9.00 encantration of a H.SO, solution if 15 ml of the
[index] [4835] [2344] [1013] [1420] [2204] [7065] [6598] [3552] [3763] [8185]
In this video we will look at the equation for HCl + H2O and write the products. When we add HCl to H2O the HCl will dissociate and break into H+ and Cl-. ... 069 - pH and BuffersIn this video Paul Andersen explains how buffer solutions maintain pH in a solution. A buffer solution is made up of a weak acid and its... In this video we will describe the equation NaOH + H2O and write what happens when NaOH is dissolved in water.When NaOH is dissolved in H2O (water) it will d... This video gives u a key concept to find most acidic hydrogen in a molecule.To support me in my journey you can donate (Paytm@ 7870993388).A small amount of ... In this video we will describe the equation K2CO3 + H2O and write what happens when K2CO3 is dissolved in water.When K2CO3 is dissolved in H2O (water) it wil... In this video we will describe the equation CH3OH + H2O and write what happens when CH3OH is mixed with water.When CH3OH is mixed with H2O (water) there isn’... This chemistry video tutorial shows you how to identify an ionic compound as acidic, basic, or a neutral salt. You need to know the 6 common strong acids su... If you're in high school or college taking Chemistry, I can help you understand everything you need to know so you can pass! On this YouTube channel, you'll find video tutorials on all of the ... Identify the Lewis acid and Lewis base in the following reaction:SO3 + H2O ⇌ H2SO4 This video on acids and bases shows you how to calculate the pH, pOH, [H+], [OH-] of acid and base solutions. Acids and bases can be measured by their stren...
Copyright © 2024 m.game-and-money.site